SAGITTARIUS consortium
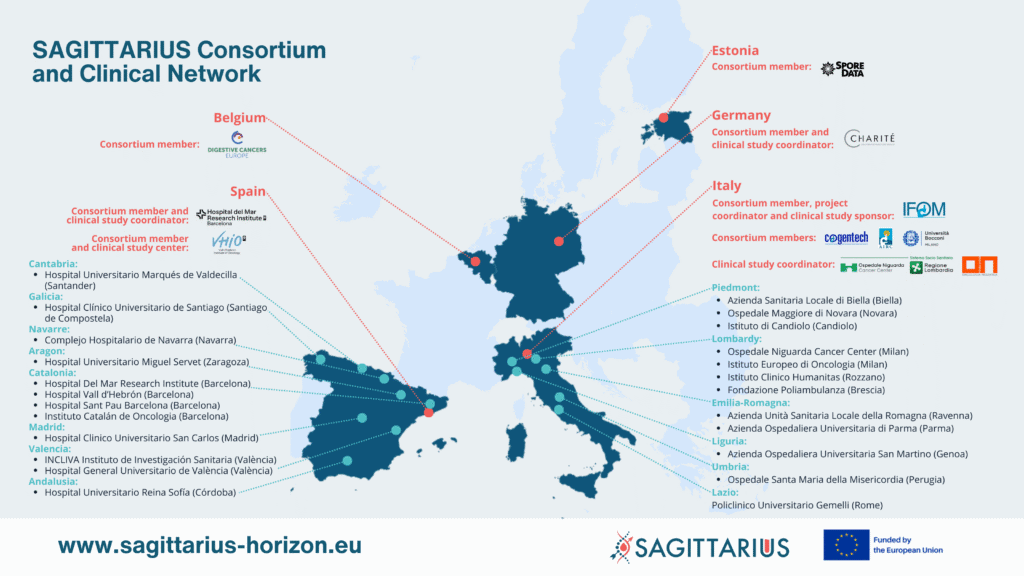
SAGITTARIUS is a large and multifaceted clinical trial project. It is being led by a highly multidisciplinary consortium of data scientists, health and social care experts from 5 different European countries - Italy, Belgium, Estonia, Spain, and Germany. The Consortium works alongside a network of highly qualified cancer centres in Italy, Spain and Germany where the SAGITTARIUS approach will be clinically tested.
The Consortium
The consortium behind the SAGITTARIUS project consists of 8 partners carefully selected on the basis of their specific competences and potential contribution to the project objectives.
Partners

IFOM - Istituto Fondazione di Oncologia Molecolare Ets, Italy
Design and management and logistics of SAGITTARIUS clinical trial. Overall project management.
➼ Website

IFOM affiliated partner.
Tumor genetic profiling.
➼ Website

AIRC ETS - Fondazione Airc per la ricerca sul cancro Ets, Italy
Stakeholder engagement, communication, and dissemination of materials.
➼ Website

Hospital del Mar Research Institute Barcelona, Spain
Clinical trial execution.
➼ Website


Stakeholder engagement, communication, and dissemination of materials.
➼ Website

Sporedata OU, Estonia
Integrated data analysis.
➼ Website